Calculate The Change In Internal Energy For A System That Is Giving Off 250 Kj
Calculate the change in internal energy for a system that is giving off 250 kj. Calculate the change in internal energy ΔE for a system that is giving off 250 kJ of heat and is changing from 1200 L to 600 L in volume at 150 atm pressure. 2 C8H18 25 O2 16 CO2 18 H2O ΔHrxn -11018 kJ. Chemistry questions and answers.
Work PdV View the full answer Previous question Next question. Calculate the change in internal energy E for a system that is giving off 250kJ of heat and is changing from 1800L to 1500L in volume at 150atm pressure. Using the following equation for the combustion of octane calculate the heat of reaction for 1000 g of octane.
Calculate the change in internal energy Δ E for a system that is giving off 250 kJ of heat and is changing from 1800 L to 1500 L in volume at 150 atmpressure. Calculate the change internal energy ΔE for a system that is giving off 650 kJ of heat and is performing 855 J of work on the surroundings asked Sep 20 2016 in. Q and w are path functions because.
1013 J 1 Latm. Remember that 1013 J 1 Latm. It depends only on the initial and final states of the system and not on the path of change.
The change in internal energy ΔE is a state function because. Remember that 1013 J 1 Latm13______A259 kJB937 kJC-241 kJD-160 kJE-259 kJ. Chemistry questions and answers.
Calculate the change in internal energy E for a system that is giving off 250 kJ of heat and is changing from 1200 L to 600 L in volume at 150 atm pressure. Where ΔH is the heat exchanged between a system and its surroundings ΔU is the total change in internal energy of a system ΔnRT is the work done by or on the system. Calculate the change in internal energy ΔE for a system that is giving off 250 kJ of heat and is changing from 1800 L to 1500 L in volume at 150 atm pressure.
System that is giving off 250 kJ of heat - 250 kj energy lost by system 1200 L system decreases to 600 L at 150 atm pressure. Calculate the change in internal energy ΔE for a system that is giving off 250 kJ of heat and is changing from 2400 L to 2100 L in volume at 150 atm pressure.
Calculate the change in internal energy Δ E for a system that is giving off 250 kJ of heat and is changing from 1800 L to 1500 L in volume at 150 atmpressure.
Calculate the change in internal energy δe for a system that is giving off 250 kj of heat and is changing from 1200 l to 60 0 l in volume at 150 atm pressure. Remember that 1013 J 1 Latm -255 kJ. Chemistry questions and answers. Calculate the change in internal energy ΔE for a system that is giving off 250 kJ of heat and is changing from 1800 L to 1500 L in volume at 150 atm pressure. Given ΔH2Kcal8400J W500J SoΔU84005007900J. Calculate the change in internal energy δe for a system that is giving off 250 kj of heat and is changing from 1200 l to 60 0 l in volume at 150 atm pressure. Remember that 1013 J 1 Latm. Calculate the change in internal energy ΔE for a system that is giving off 250 kJ of heat and is changing from 1200 L to 600 L in volume at 150 atm pressure. Where ΔH is the heat exchanged between a system and its surroundings ΔU is the total change in internal energy of a system ΔnRT is the work done by or on the system.
Chemistry questions and answers. Given ΔH2Kcal8400J W500J SoΔU84005007900J. Calculate the change in internal energy ΔE for a system that is giving off 250 kJ of heat and is changing from 1200 L to 600 L in volume at 150 atm pressure. Remember that 1013 J 1 Latm13______A259 kJB937 kJC-241 kJD-160 kJE-259 kJ. W PΔV 159 135 atmL 135 01013 kJ 137 kJ E Q w 25 137 2363 kJ There is a 2363 kJ decrese in internal energy. Calculate the change in internal energy E for a system that is giving off 250kJ of heat and is changing from 1800L to 1500L in volume at 150atm pressure. W PΔV 159 135 atmL 135 01013 kJ 137 kJ E Q w 25 137 2363 kJ There is a 2363 kJ decrese in internal energy.


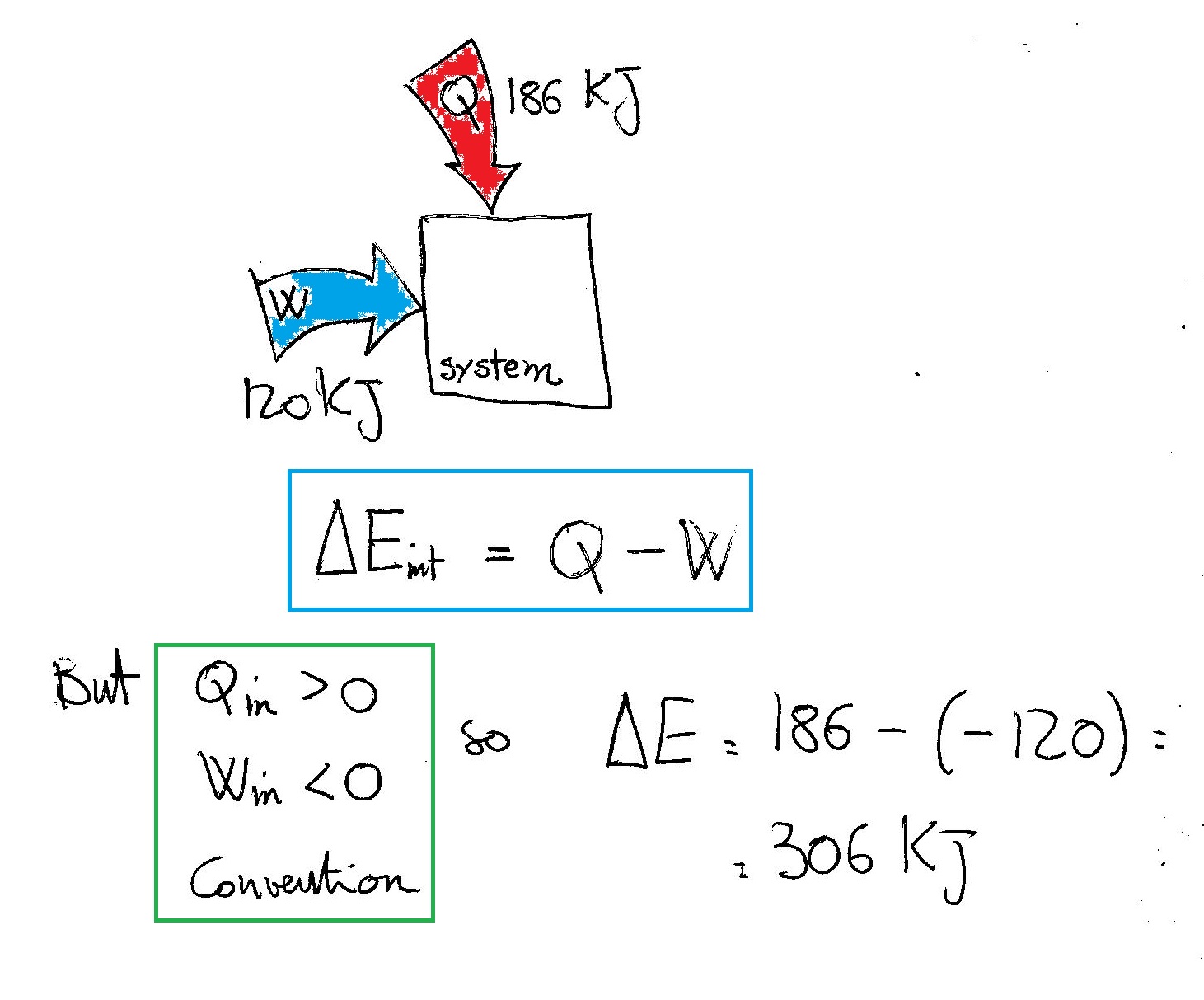










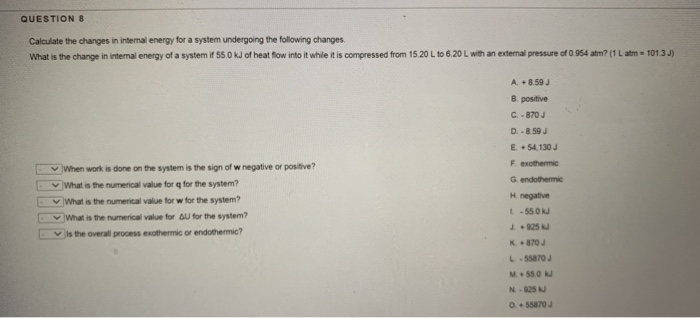


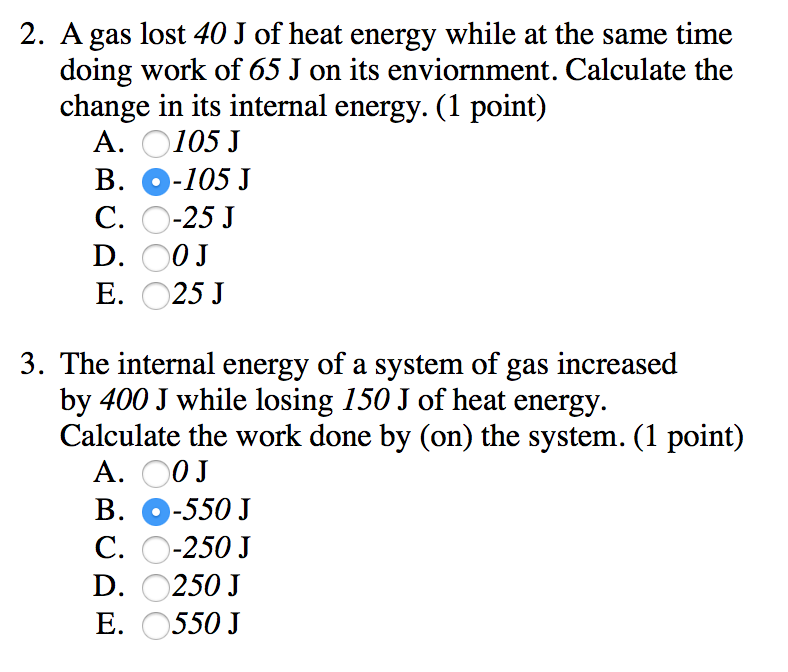






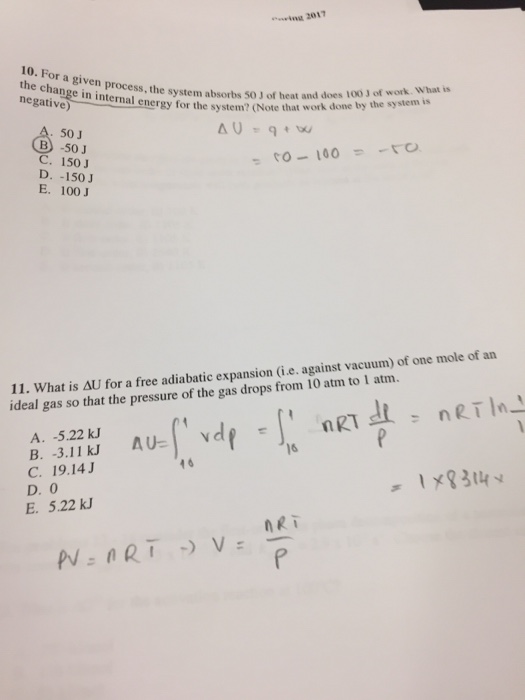















Post a Comment for "Calculate The Change In Internal Energy For A System That Is Giving Off 250 Kj"